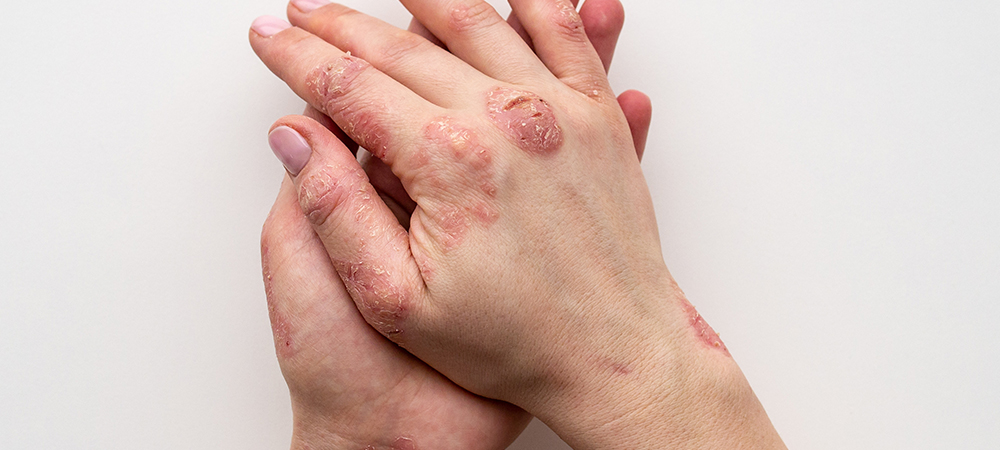
DuPont has announced the introduction of new low-cyclosiloxane silicone elastomer blends and silicone resin blends in response to growing consumer desires for products that address a range of troubling skin conditions.
These new topical excipient blends also address the market’s need to comply with evolving REACH regulatory compliance demands and to support the shared commitment of DuPont and its customers to the United Nations Sustainable Development Goals (SDGs), particularly the third one: Good Health and Well Being, which calls for protecting human health and well-being for all people at all ages.
“Over-the-counter topical and medical products to address scars, actinic keratosis, atopic dermatitis, psoriasis and other skin conditions are proliferating to meet consumer desires to feel good at every age without compromising skin protection and drug delivery efficiency,” said Michele Vigliotti, EMEA Regional Marketing Leader, DuPont. “Meanwhile, regulations on consumer healthcare products continue to evolve to ensure safety and minimise public risk.
“DuPont serves a broad spectrum of consumer and medical topical healthcare applications, from over-the-counter to prescription, ” Vigliotti said. “Whether customers are looking for technological expertise, regulatory support, or a broad selection of high-quality topical excipients that are easy to process andpatient-friendly, we are here to support them.”
Click below to share this article