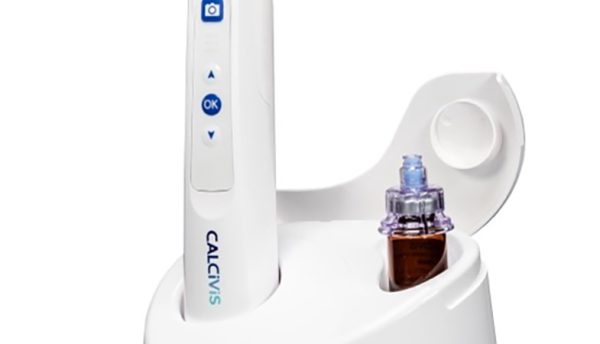
Calcivis is launching its groundbreaking new preventive dental technology in the United States. Calcivis filed a PMA supplement with the FDA for enhancements to its imaging system last year and the new ergonomic, wireless, handheld imaging device is now approved for commercialisation.
The Calcivis Imaging System will revolutionise caries management through early detection which allows for a more preventive treatment model. The ability to visualise active demineralisation (an early indicator of decay) ‘live’ as it happens on patients’ teeth, provides crucial insight as to whether a caries lesion is likely to progress and requires treatment.
The imaging system applies a patented photoprotein which, in the presence of free calcium ions released from an actively decaying tooth surface, produces a very short, low level light flash. An integrated intra-oral sensor within the Calcivis imaging device immediately detects the luminescence (light flash) and presents clinicians with a chairside demineralisation map. The Calcivis Imaging System is safe and effective for use in adults and children 6 years of age or older and can be used on all accessible coronal tooth surfaces.
Adam Christie, CEO at Calcivis, said: “With our US commercial team in place and a very user-friendly, ready for market device, we are confident that the Calcivis Imaging System will significantly improve patient care and enable restorative dentistry to move to a more preventive approach.”
The US roll out will begin with a limited release launch in the greater Boston area in early 2024.
Click below to share this article